Introduction: ATHN 8: U.S. Cohort Study of Previously Untreated Patients (PUPs) with Congenital Hemophilia is sponsored by the American Thrombosis and Hemostasis Network (ATHN) and is being conducted at ATHN-affiliated sites in the United States. The ATHN 8: PUPs Study collects detailed demographic, diagnosis, treatment, bleed, and inhibitor data on children with moderate and severe hemophilia born on or after January 1, 2010 and followed at an ATHN-affiliated hemophilia treatment center (HTC). The endpoint for the overall study is inhibitor development or achieving 50 exposure days. A confirmed inhibitor is defined as two consecutive positive inhibitor titers (>0.5 Nijmegen Bethesda Units (BU) for hemophilia A and >0.3 Nijmegen BU for hemophilia B) which results in change in treatment. Available family history is also collected. For this interim analysis, we hypothesized that children with severe hemophilia A (HA) and a family history (FH) of hemophilia would have earlier age of diagnosis.
Methods: Available data through April 30, 2020 were analyzed for participants with severe HA (factor VIII <1%) born between January 1, 2010 and December 31, 2019. Diagnostic history, circumcision, and bleeding and treatment within the first 30 days of life were compared between participants with and without a known FH.
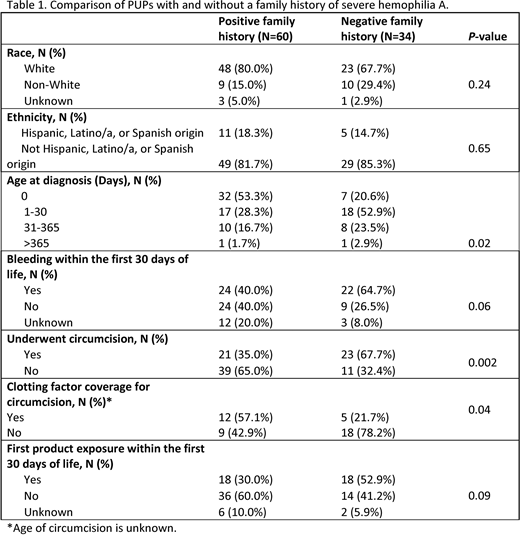
Results: Twenty-six HTCs have enrolled 112 male participants with severe HA. FH regarding hemophilia is known in 94 (84%) and positive in 60 (64%). Patients with a positive FH compared to patients without a positive FH were diagnosed significantly earlier and were less likely to have circumcision (Table 1). Bleeding rate and factor exposure rate was similar within the first 30 days of life. Among 46 subjects who bled within the first 30 days of life, 6 (13.0%) bled due to circumcision (2 have a positive family history, and 4 have a negative family history); 34/46 (74%) had other bleeding events within the first 30 days.
Conclusions: The interim analysis of PUPs born with severe HA between 2010-2019 demonstrates a majority have a FH of HA which is associated with an earlier age at diagnosis compared to those without a FH. Those without a FH of HA have higher rates of circumcision. Earlier identification of hemophilia could result in perinatal management strategies and avoidance of procedures such as circumcision that could result in bleeding.
Thornburg:NovoNordisk: Research Funding; Genentech: Speakers Bureau; American Thrombosis and Hemostasis Network: Research Funding; National Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Pharmaceuticals: Research Funding; Spark Therapeutics: Consultancy; Sanofi Genzyme: Consultancy, Other: Data Safety Monitoring Board, Research Funding; Bluebird Bio: Consultancy; Ironwood Pharmaceuticals: Consultancy, Other: Data Safety Monitoring Board; Biomarin: Consultancy, Speakers Bureau. Friedman:Alexion: Speakers Bureau; Instrumentation Laboratories: Consultancy; Alexion: Consultancy; Bayer: Consultancy. Guerrera:NovoNordisk: Consultancy, Speakers Bureau; Kedrion: Consultancy; Biomarin: Speakers Bureau; Sanofi Genzyme: Speakers Bureau; Takeda: Consultancy. Malec:SOBI: Consultancy; Bayer: Consultancy; CSL: Consultancy; Takeda: Consultancy; Sanofi Genzyme: Consultancy, Research Funding, Speakers Bureau. Simpson:HEMA Biologics: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Speakers Bureau; CSL Behring: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Bioverativ/Sanofi: Research Funding. Tarantino:Spark: Membership on an entity's Board of Directors or advisory committees; HRSA: Membership on an entity's Board of Directors or advisory committees; CDC: Membership on an entity's Board of Directors or advisory committees; Dova: Membership on an entity's Board of Directors or advisory committees; Pfizer: Other; NovoNordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Research Funding; Octapharma: Membership on an entity's Board of Directors or advisory committees; Grifols: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Sobi: Membership on an entity's Board of Directors or advisory committees; Biomarin: Membership on an entity's Board of Directors or advisory committees. Carpenter:Novo Nordisk: Honoraria; Shire: Research Funding; Genentech, Inc.: Honoraria; Kedrion: Honoraria; CSL Behring: Research Funding; Hemostasis & Thrombosis Research Society: Membership on an entity's Board of Directors or advisory committees; American Academy of Pediatrics: Other: PREP Heme/Onc editorial board; American Thrombosis and Hemostasis Network: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal